Revealing the thermodynamic driving force for ligand-based reductions in quinoids; conceptual rules for designing redox active and non-innocent ligands†
Abstract
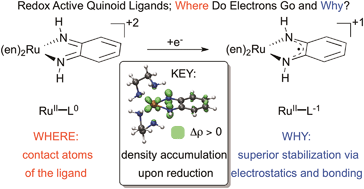
Metal and ligand-based reductions have been modeled in octahedral ruthenium complexes revealing metal–ligand interactions as the profound driving force for the redox-active behaviour of orthoquinoid-type ligands. Through an extensive investigation of redox-active ligands we revealed the most critical factors that facilitate or suppress redox-activity of ligands in metal complexes, from which basic rules for designing non-innocent/redox-active ligands can be put forward. These rules also allow rational redox-leveling, i.e. the moderation of redox potentials of ligand-centred electron transfer processes, potentially leading to catalysts with low overpotential in multielectron activation processes.

 Please wait while we load your content...
Please wait while we load your content...