Solid base catalysed 5-HMF oxidation to 2,5-FDCA over Au/hydrotalcites: fact or fiction?†
Abstract
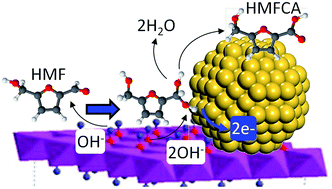
Nanoparticulate gold has emerged as a promising catalyst for diverse mild and efficient selective aerobic oxidations. However, the mechanism of such atom-economical transformations, and synergy with functional supports, remains poorly understood. Alkali-free Mg–Al hydrotalcites are excellent solid base catalysts for the aerobic oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furan dicarboxylic acid (FDCA), but only in concert with high concentrations of metallic gold nanoparticles. In the absence of soluble base, competitive adsorption between strongly-bound HMF and reactively-formed oxidation intermediates site-blocks gold. Aqueous NaOH dramatically promotes solution phase HMF activation, liberating free gold sites able to activate the alcohol function within the metastable 5-hydroxymethyl-2-furancarboxylic acid (HMFCA) reactive intermediate. Synergistic effects between moderate strength base sites within alkali-free hydrotalcites and high gold surface concentrations can afford highly selective and entirely heterogeneous catalysts for aqueous phase aldehyde and alcohol cascade oxidations pertinent to biomass transformation.
- This article is part of the themed collection: ISACS17: Challenges in Chemical Renewable Energy


 Please wait while we load your content...
Please wait while we load your content...