Unified total synthesis of the natural products endiandric acid A, kingianic acid E, and kingianins A, D, and F†
Abstract
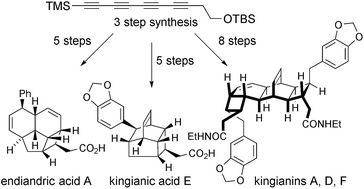
A measure of the strength of a synthetic strategy is its versatility: specifically, whether it allows structurally distinct targets to be prepared. Herein we disclose a unified approach for the total synthesis of natural products of three distinct structural types, all of which occur naturally as racemic mixtures. The point of divergence involves the terminal alkylation of a conjugated tetrayne, and culminates in a significantly shortened synthesis of endiandric acid A (8 steps), the first total synthesis of kingianic acid E (8 steps), and a second-generation synthesis of kingianins A, D, and F (11 steps). Evidence for redox catalysis in the biosynthesis of kingianic acid E is presented.

 Please wait while we load your content...
Please wait while we load your content...