Nitrite reduction by copper through ligand-mediated proton and electron transfer†
Abstract
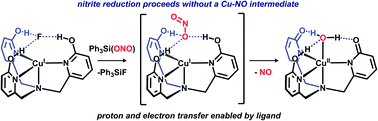
Nitrite reduction by a copper complex featuring a proton-responsive tripodal ligand is demonstrated. Gaseous nitric oxide was confirmed as the sole NOX by-product in quantitative yield. DFT calculations predict that nitrite reduction occurs via a proton and electron transfer process mediated by the ligand. The reported mechanism parallels nitrite reduction by copper nitrite reductase.

 Please wait while we load your content...
Please wait while we load your content...