Remote functionalization of hydrocarbons with reversibility enhanced stereocontrol†
Abstract
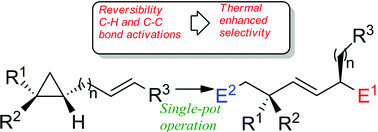
Remote functionalization of hydrocarbons could be achieved through successive zirconocene-mediated allylic C–H bond activations followed by a selective C–C bond cleavage. Determination of the reaction mechanism by density functional theory (DFT) calculations shows that the high stereocontrol observed in this process results from a large number of energetically accessible equilibria feeding a preferred reactive channel that leads to the major product. A distinctive consequence of this pattern is that stereoselectivity is enhanced upon heating.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...