A convergent total synthesis of ouabagenin†
Abstract
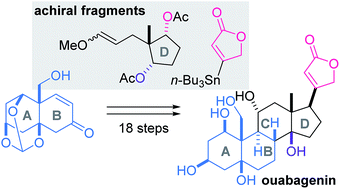
A convergent total synthesis of ouabagenin, an aglycon of cardenolide glycoside ouabain, was achieved by assembly of the AB-ring, D-ring and butenolide moieties. The multiply oxygenated cis-decalin structure of the AB-ring was constructed from (R)-perillaldehyde through the Diels–Alder reaction and sequential oxidations. The intermolecular acetal formation of the AB-ring and D-ring fragments, and combination of the intramolecular radical and aldol reactions, assembled the requisite steroidal skeleton in a stereoselective fashion. Finally, stereoselective installation of the C17-butenolide via the Stille coupling and hydrogenation led to ouabagenin.

 Please wait while we load your content...
Please wait while we load your content...