Stepwise isolation of low-valent, low-coordinate Sn and Pb mono- and dications in the coordination sphere of platinum†
Abstract
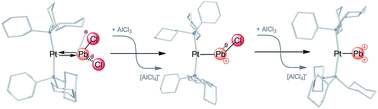
Synthetic access to low-coordinate Pb mono- and dications is in general impeded due to their poor solubility and highly electrophilic nature. However, the electrophilicity of these cations can be tamed by attaching them to electron-rich transition metals. Following this principle we have isolated low-valent Pb mono- ([(Cy3P)2Pt–PbCl]2[AlCl4]2, 8a) and dications ([(Cy3P)2Pt(Pb)][AlCl4]2, 11) in the coordination sphere of platinum. The same approach then has been implemented for the isolation of analogous low-valent Sn mono- (7a) and dications (10). An energy decomposition analysis (EDA-NOCV) was performed to investigate the nature of Pt–Pb and Pb–Cl bonding in [(Cy3P)2Pt(PbCl2)] (2), 8a and 11. The results show that the Pt–Pb bonds in 8a and 11 are electron-sharing in nature, whereas that of the precursor 2 is a dative bond. The breakdown of attractive interactions in 2, 8a and 11 reveals that the ionic interactions in the analyzed Pt–Pb and Pb–Cl bonds are always stronger than the covalent interactions, except for the Pb–Cl bond in 8a. The calculated D3 dispersion energies show that dispersion interactions play a key role in the thermodynamic stability of 2, 8a and 11.
- This article is part of the themed collection: Celebrating the Chemical Science in India - Leaders in the Field Symposium

 Please wait while we load your content...
Please wait while we load your content...