Reversible assembly of enantiomeric helical polymers: from fibers to gels†
Abstract
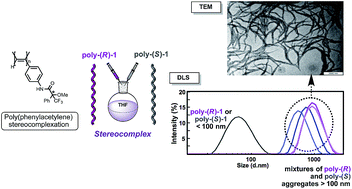
A novel class of stereocomplexes is described by the interaction of helically complementary poly(phenylacetylene)s (PPAs) carrying an α-methoxy-α-trifluoromethylphenylacetamide pendant group. The formation of the stereocomplex requires the presence of cis amide bonds on the external crest of the polymer to provide efficient cooperative supramolecular hydrogen bonding between matching enantiomeric helical structures. The interlocking of the chains gives rise to supramolecular fiber-like aggregates that, at higher concentrations, result in gels. The modification of the cis–trans amide conformation at the pendant groups allows the controlled formation and cleavage of the stereocomplex due to a dramatic change between the intermolecular and intramolecular hydrogen bond interactions.

 Please wait while we load your content...
Please wait while we load your content...