Core–shell structured Fe3O4@SiO2@CdS nanoparticles with enhanced visible-light photocatalytic activities†
Abstract
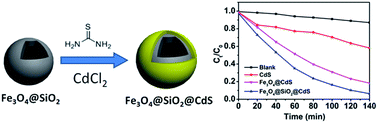
A chelating-assistant growth route of CdS on the surface of Fe3O4@SiO2 nanoparticles (NPs) was used to form a magnetically recoverable photocatalyst. Characterization by transmission electron microscopy, X-ray powder diffraction, Raman spectroscopy and a vibrating sample magnetometer reveals monodispersed superparamagnetic Fe3O4@SiO2@CdS NPs (ca. 250 nm) have been formed with a uniform CdS shell thickness of ca. 20 nm, Brunauer–Emmett–Teller (BET) surface area of ca. 25.1 m2 g−1 and saturation magnetization of 22.02 emu g−1. This composite shows excellent photocatalytic activity towards the degradation of methylene blue (MB) under visible-light irradiation with a reaction constant of 1.95 × 10−2 min−1 in spite of the low weight percentage of CdS (9.15%) as determined by the energy-dispersive X-ray spectroscopy, which is higher than those observed on Fe3O4@CdS (53.30%, 1.22 × 10−2 min−1) and CdS NPs (3.33 × 10−3 min−1). Furthermore, Fe3O4@SiO2@CdS can be quickly magnetically recovered within 30 s by applying an external magnetic field near the solution after the photocatalytic process, which still preserves the excellent particle monodispersity with the slightly reduced CdS thickness (ca. 15 nm), while Fe3O4@CdS and CdS NPs are severely photo-corroded and aggregated. The maximized specific surface area from uniform coating and the efficient generation of activated oxygen species from CdS shells might be responsible for the enhanced photoactivity.

 Please wait while we load your content...
Please wait while we load your content...