One-step electrodeposition fabrication of a superhydrophobic surface on an aluminum substrate with enhanced self-cleaning and anticorrosion properties
Abstract
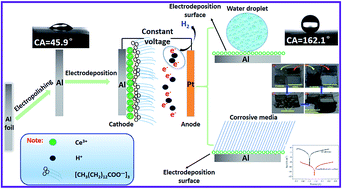
This paper presents a facile, low-cost, one-step approach to fabricate a superhydrophobic surface via electrodepositing aluminum in an ethanol solution containing cerium nitrate hexahydrate and myristic acid. The wettability, morphology and chemical composition of the as-prepared surfaces were characterized by water contact angle (WCA), scanning electron microscopy (SEM), energy dispersive spectrometry (EDS), and Fourier transform infrared spectroscopy (FTIR). The highest WCA of the as-prepared surface after the one-step electrodeposition process reaches 162.1°. Water droplet adhesion force, water stream reflecting, water droplet bouncing, and self-cleaning properties were investigated respectively. Potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) tests demonstrated that the as-prepared superhydrophobic surface greatly improved the corrosion resistance of the aluminum substrate. The corrosion current density (Icorr) of the as-prepared superhydrophobic surface is smaller by more than 3 orders of magnitude. The presented method is facile, low-cost, and relatively environmentally friendly, and has promising applications in anticorrosion and anticontamination fields.

 Please wait while we load your content...
Please wait while we load your content...