Synthesis, 64Cu-labeling and PET imaging of 1,4,7-triazacyclononane derived chelators with pendant azaheterocyclic arms†‡
Abstract
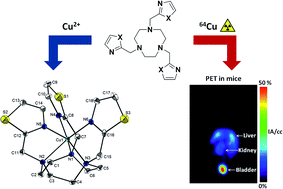
The TACN derived chelators NOTI, NOTI-Me and NOTThia were synthesized in a facile, single reaction step. The new chelators were readily labeled with 64Cu under mild conditions over a wide pH range. The corresponding 64Cu complexes are of high stability in vitro as determined by Cu2+ exchange experiments, acid decomplexation and serum stability studies. Single crystal X-ray analysis showed that the six-coordinate copper complexes exhibited a distorted prismatic CuN3N′3 coordination geometry similar to Cu(NOTA). Biological testing by small animal PET imaging revealed retention of 64Cu complexes in the kidneys and, to a lesser extent, in the liver. Altogether, the results presented support the development of bifunctional derivatives for conjugation to targeting vectors and further testing in vivo.

 Please wait while we load your content...
Please wait while we load your content...