Fabrication of a non-enzymatic Ni(ii) loaded ZSM-5 nanozeolite and multi-walled carbon nanotubes paste electrode as a glucose electrochemical sensor†
Abstract
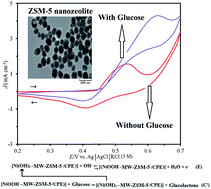
Effective electro-oxidation of glucose is critically important in developing analytical sensors and carbohydrate-based fuel cells. In this study, a template-free ZSM-5 nanozeolite was synthesized hydrothermally with spherical particle diameters of 40–60 nm, as characterized by scanning electron microscopy. Then, a carbon paste electrode (CPE) was modified by multi-walled carbon nanotubes (MWCNTs), ZSM-5 nanozeolite and Ni2+ ions (Ni–MW–ZSM-5/CPE). Electrochemical studies of this electrode were performed using cyclic voltammetry which exhibits the redox behavior of the Ni(III)/Ni(II) couple in alkaline medium. This modified electrode was used as an anode for the electrocatalytic oxidation of glucose in 0.1 mol L−1 NaOH solution. The results confirmed that ZSM-5 nanozeolite at the surface of the CPE improved the catalytic efficiency of the dispersed nickel ions toward glucose oxidation. The values of electron transfer coefficient, electrode surface coverage and charge-transfer rate constant for Ni(III)/Ni(II) redox couple were found to be 0.65, 4.04 × 10−8 mol cm−2 and 0.184 s−1, respectively. Also, the diffusion coefficient and the mean value of the catalytic rate constant for glucose and redox sites of the electrode were found to be 1.66 × 10−4 cm2 s−1 and 1.136 × 108 cm3 mol−1 s−1, respectively. The sensor showed an acceptable linear range of 0.5–6.1 mM with a detection limit of 0.14 mM (S/N = 3) by cyclic voltammetry technique. Moreover, differential pulsed voltammetry method revealed a linear range of 0.0001–0.01 mM with a detection limit of 3.5 × 10−5 mM. Based on the results, the fabricated electrode (Ni–MW–ZSM-5/CPE) showed good catalytic activity, good stability, high sensitivity and reproducibility.

 Please wait while we load your content...
Please wait while we load your content...