Facile hydrothermal synthesis and formation mechanisms of Bi2Te3, Sb2Te3 and Bi2Te3–Sb2Te3 nanowires†
Abstract
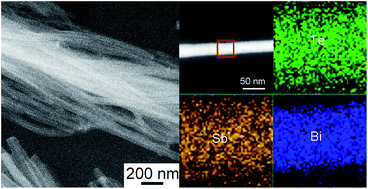
A facile and scalable glucose-assisted hydrothermal method has been established for the fabrication of Bi2Te3, Sb2Te3 and Bi2Te3–Sb2Te3 nanowires in high yield. PH additives, such as HCl and NaOH, play crucial roles for the fabrication of Bi2Te3, Sb2Te3 and Bi2Te3–Sb2Te3 nanowires. It is suggested that fine Te nanowires are initially formed and they serve as templates for the fabrication of telluride nanowires. Bi2Te3 nanowires are obtained by direct one-step hydrothermal synthesis under acidic conditions when HCl is used as the pH additive. The as-prepared Bi2Te3 nanowires have different wire axes that can be parallel or perpendicular to the [001] direction. Two different mechanisms, i.e., structural preference growth mechanism and coherent growth mechanism are suggested for the formation of Bi2Te3 nanowires. A single phase of Sb2Te3 cannot be obtained under acidic conditions due to the slow kinetics of the reduction reaction even at elevated reaction temperatures. A two-step synthesis is proposed for the fabrication of Sb2Te3 and Bi2Te3–Sb2Te3 nanowires, where Te nanowires are first formed with a HCl additive and Sb2Te3 and Bi2Te3–Sb2Te3 nanowires are then fabricated with a NaOH additive. Besides the enhanced phonon scattering and quantum confinement effect, the structure and composition fluctuation in the Bi2Te3–Sb2Te3 nanowires may further adjust the energy band structure and improve the thermoelectric properties.

 Please wait while we load your content...
Please wait while we load your content...