Oxidative oxygen-nucleophilic bromo-cyclization of alkenyl carbonyl compounds without organic wastes using alkali metal reagents in green solvent†
Abstract
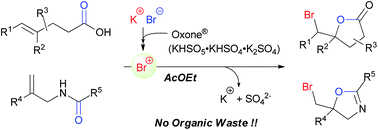
A bromo-lactonization of alkenyl carboxylic acids and a bromo-cyclization of N-allyl amides as oxygen-nucleophilic bromo-cyclization reactions were developed via the oxidative umpolung of bromide using alkali metal bromide and inorganic oxidant to provide the corresponding cyclization products in high yields. In particular, the use of AcOEt, the solvent of choice for green sustainable reactions, led to the high reactivities of the present reactions. This methodology is highly recommended for green sustainable chemistry because it uses stable and non-hazardous reagents instead of other bromo reagents and oxidants, and does not produce organic wastes that pollute the environment.

 Please wait while we load your content...
Please wait while we load your content...