Conversion of carbohydrates into 5-hydroxymethylfurfural in an advanced single-phase reaction system consisting of water and 1,2-dimethoxyethane
Abstract
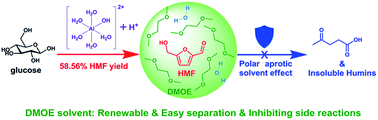
5-Hydroxymethylfurfural (HMF) is a bio-based platform chemical that may be converted into various chemicals and fuels. In the present study, we developed an advanced low-boiling single-phase reaction system for producing HMF from glucose. It consists of water and 1,2-dimethoxyethane (DMOE) and uses AlCl3 as catalyst. Our results show that introduction of DMOE can substantially enhance HMF production because of the polar aprotic solvent effect provided by DMOE. Under optimal conditions, a high HMF yield (58.56%) was obtained. GC-MS of the liquid-phase products revealed that HMF and furans comprised 80% and ∼90% of the detected products. Formation of liquid-phase products, including furans, oxygenated aliphatics, cyclopenten-1-ones, and pyrans is discussed. Further study of the humins formed during glucose conversion showed the effective inhibition of humin formation by DMOE. The structure of humins was characterized by FTIR spectroscopy. Finally, HMF production from disaccharides (sucrose, maltose and cellobiose) and polysaccharide (cellulose) using the water–DMOE system resulted in good yields, demonstrating that our single-phase water–DMOE solvent system has good potential use in HMF production from glucose and complex carbohydrates.

 Please wait while we load your content...
Please wait while we load your content...