Highly selective one pot synthesis and biological evaluation of novel 3-(allyloxy)-propylidene acetals of some natural terpenoids†
Abstract
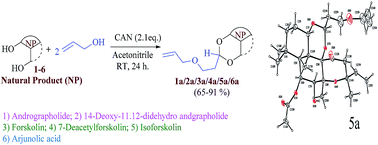
A series of 3-(allyloxy)-propylidene acetals 1a to 6a of some natural terpenoids like andrographolides 1, 2, forskolins 3–5 and arjunolic acid 6 were developed by a novel one pot synthetic strategy using ceric ammonium nitrate as a catalyst. The method is both chemo and regioselective towards 1,3-acetal formation without affecting other poly functional groups of terpenoids. O-Allylation is an important functional group transformation for alcohols and the resulting end allylic double bond may participate in a number of synthetically useful transformations like olefin metathesis. Acetal of andrographolide 1a was further converted into 1b, 1c and 1d by dimerization, acetylation and epoxidation respectively. All the synthesized compounds were screened for in vitro antiproliferative activity against four cancer cell lines B16F10, THP-1, PC-3 and SKOV3. Derivatives of andrographolide 1a, 1b, 1c and 2a, forskolin 4a and arjunolic acid 6a have shown promising cytotoxicity (IC50 < 10 μg ml−1) in most of the tested cell lines. Also compounds 1b (IC50 0.83 μg ml−1) and 5a (IC50 3.43 μg ml−1) showed significant α-glucosidase inhibition in an in vitro assay. Structures of all the synthesized compounds were confirmed by NMR, mass and IR spectral data. A single crystal X-ray analysis of 5a also confirmed the 3-(allyloxy)-propylidene acetal formation.

 Please wait while we load your content...
Please wait while we load your content...