Diversity-oriented reconstruction of primitive diketopiperazine-fused tetrahydro-β-carboline ring systems via Pictet–Spengler/Ugi-4CR/deprotection-cyclization reactions†
Abstract
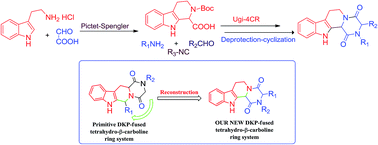
An expedient construction of tetrahydro-β-carbolinediketopiperazine ring systems, which are present in various indole alkaloids, is documented. The synthetic strategy proceeds through a Pictet–Spengler reaction followed by an Ugi-4CR and deprotection-cyclization reactions. This is the first report of a Pre-Ugi multicomponent reaction modification using 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-carboxylic acid as the acidic partner. This novel approach provides highly diverse reconstructions of novel tetrahydro-β-carbolinediketopiperazine derivatives, which can also be used as privileged structures in medicinal chemistry.

 Please wait while we load your content...
Please wait while we load your content...