An ortho-quinone methide based strategy towards the rubromycin spiroketal family†
Abstract
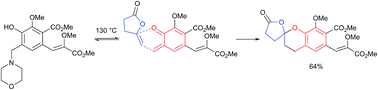
A method for the generation/in situ hetero-Diels–Alder cycloaddition of a trisubstituted ortho-quinone methide (o-QM) is described. Heating of ortho-hydroxybenzylamines was found to be a more effective strategy for the formation of o-QMs than use of the corresponding N-substituted quaternary salts hitherto employed. This method was used to synthesise the spiroketal core of the rubromycins.

 Please wait while we load your content...
Please wait while we load your content...