Electrochemical and magnetic properties of nanostructured CoMn2O4 and Co2MnO4†
Abstract
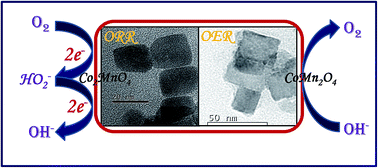
In this study, we have focused on the synthesis of cobalt manganite nanostructures using a simplistic hydrothermal route. We have explored these spinels as alternative low-cost bifunctional electrocatalysts for oxygen reduction/evolution reactions (ORR/OER). Herein, we have developed energy-saving, facile and rapid synthetic methodologies for highly active spinel electrocatalysts. Two spinel phases, cubic Co2MnO4 and tetragonal CoMn2O4 have been successfully obtained by tuning the stoichiometric ratio of Co and Mn salts respectively. These CoMn2O4 and Co2MnO4 nanocubes have been used as bifunctional catalysts towards OER and ORR. Electrocatalytic experiments show that cubic Co2MnO4 nanocubes show five times higher activity towards ORR than tetragonal CoMn2O4 nanocubes while the tetragonal phase is a better electrocatalyst towards OER than the cubic Co2MnO4 phase. XPS studies revealed two types of oxygen (lattice O and surface adsorbed O species like OH−) and the efficiency of the catalyst could be related to the binding affinity of oxygen. This explains the better catalytic activity of cubic Co2MnO4 which has a large percentage of adsorbed oxygen species. The stability of the catalyst was confirmed by carrying out TEM studies on a sample after carrying out 25 cycles. Magnetization experiments reveal that both the tetragonal CoMn2O4 as well as cubic Co2MnO4 show hysteresis at 10 K and 100 K without reaching saturation, which confirms an existing ferrimagnetic order in the samples. Both the tetragonal and cubic phases show Tc ∼ 110 K and 150 K respectively.

 Please wait while we load your content...
Please wait while we load your content...