Understanding the high reactivity of carbonyl compounds towards nucleophilic carbenoid intermediates generated from carbene isocyanides
Abstract
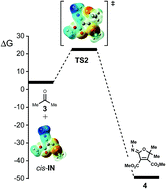
The high reactivity of carbonyl compounds towards the carbenoid intermediate cis-IN, generated in situ by the addition of methyl isocyanide to dimethyl acetylenedicarboxylate (DMAD), has been investigated at the MPWB1K/6-311G(d,p) computational level by using Molecular Electron-Density Theory (MEDT). This multicomponent (MC) reaction is a domino process that comprises two sequential reactions: (i) the formation of a nucleophilic carbenoid intermediate trans-IN; and (ii) the nucleophilic attack of cis-IN on the carbonyl compound, resulting in the formation of the final 2-iminofuran derivative. The present MEDT study establishes that the high nucleophilic character and the electronic structure of the carbenoid intermediate, cis-IN, together with the specific approach mode of the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond during the nucleophilic attack of the sp2 hybridised carbenoid C4 center of cis-IN on the carbonyl C5 carbon of acetone, enables the formation of the C4–C5 single bond with a very low activation enthalpy, 3.3 kcal mol−1, without any external electrophilic activation of the carbonyl group, and the subsequent ring closure through the downhill formation of the C–O single bond. The Bonding Evolution Theory (BET) study for the formation of the 2-iminofuran allows characterisation of the mechanism as a [2n + 2n] cycloaddition, ruling out the proposed 1,3-dipolar cycloaddition mechanism.
O double bond during the nucleophilic attack of the sp2 hybridised carbenoid C4 center of cis-IN on the carbonyl C5 carbon of acetone, enables the formation of the C4–C5 single bond with a very low activation enthalpy, 3.3 kcal mol−1, without any external electrophilic activation of the carbonyl group, and the subsequent ring closure through the downhill formation of the C–O single bond. The Bonding Evolution Theory (BET) study for the formation of the 2-iminofuran allows characterisation of the mechanism as a [2n + 2n] cycloaddition, ruling out the proposed 1,3-dipolar cycloaddition mechanism.

 Please wait while we load your content...
Please wait while we load your content...