Silybin, a flavonolignan from milk thistle seeds, restrains the early and advanced glycation end product modification of albumin
Abstract
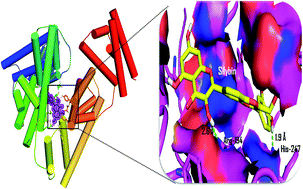
We report the inhibitory activity of silybin, a flavonolignan against the formation of early and advanced glycation end products based on fluorescence, circular dichroism and molecular interaction studies. Silybin was found to be a potent inhibitor for both the early and advanced glycation end products. The inhibition of glycation mediated fibrillation of BSA was determined based on the binding of amyloid specific dyes i.e. thioflavin T, congo red and confocal imaging. Also, we determined the protective effect of silybin towards the oxidative damage induced hemolysis of erythrocytes. The molecular interaction pattern of silybin revealed its better affinity with BSA when compared to the other natural inhibitors of glycation such as quercetin, epigallocatechin gallate, and curcumin.

 Please wait while we load your content...
Please wait while we load your content...