Solvothermal synthesis of 1D nanostructured Mn2O3: effect of Ni2+ and Co2+ substitution on the catalytic activity of nanowires†
Abstract
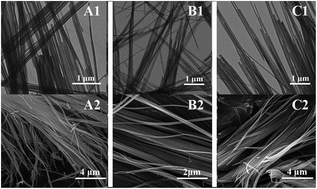
In this paper, cation modified one dimensional Mn2O3 nanowires were synthesized via a solvothermal synthesis and calcination free from the template-assisted method. The samples were characterized in detail by transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), Raman spectroscopy, high resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS). XRD results revealed the homogeneity of Ni–Mn–O/Co–Mn–O solid solutions. By introducing the two different cations the Mn2O3 nanowires can be freely manipulated. The H2-TPR measurement showed the enhanced reduction behaviors of the doped manganese oxide (Mn2O3) samples. The presence of Ni2+ and Co2+ produced lattice defects and promoted the production of oxygen vacancies, which explained the results that Ni2+/Co2+ doped Mn2O3 showed higher catalytic activity than the pure sample.

 Please wait while we load your content...
Please wait while we load your content...