Physicochemical properties of novel cholinium ionic liquids for the recovery of silver from nitrate media
Abstract
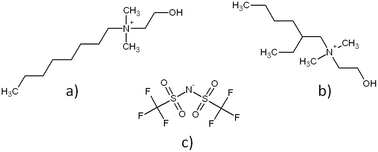
Cholinium based ionic liquids (IL), i.e. N-(2-hydroxyethyl)-N,N-dimethyl-N-octylammonium bis(trifluoromethanesulfonyl)imide ([C8linChol]+[NTf2]−) and N-(2-hydroxyethyl)-N-(2-ethylhexyl)-N,N-dimethylammonium bis(trifluoromethanesulfonyl)imide ([C8ramChol]+[NTf2]−) were synthesized and characterized by 1H NMR, 13C NMR, ATR-FTIR and ESI-MS. Activation energy of the viscous flow, molar volume, molar entropy, lattice potential energy and isobaric expansion coefficients were deduced from density and viscosity measurements. These calculations led to the conclusion that neat [C8ramChol]+[NTf2]− exhibits a slightly more compact ion arrangement than [C8linChol]+[NTf2]−. After characterizing physicochemical properties of these two neat ILs, extractive properties of [C8linChol]+[NTf2]− and [C8ramChol]+[NTf2]− saturated with water were also evaluated for silver recovery from aqueous nitrate solutions. Investigation of Ag(I) extraction as a function of pH showed a maximum of extraction efficiency at pH 5 (98.6%) for [C8linChol]+[NTf2]− and good extraction selectivity of Ag(I) and Cu(II) towards Fe(III). High stripping efficiency was achieved by using 0.44 mol L−1 nitric acid.

 Please wait while we load your content...
Please wait while we load your content...