Solvent assisted structural diversity: supramolecular sheet and double helix of a short aromatic γ-peptide†
Abstract
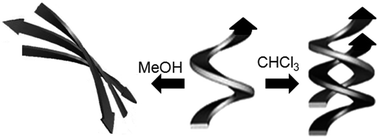
Solvent interaction has a significant effect on the folding and structural diversity of short aromatic γ-peptides that lead to a change in initial helical conformation. The internal rigidity of building blocks promotes the helical conformations of the γ-peptides containing m-aminobenzoic acid and N, N′-dicyclohexylurea. Various solution state NMR experiments show the existence of intermolecular hydrogen bonded structures of the short aromatic γ-peptides. In a polar protic solvent (MeOH), the helical strand interacts with the solvent molecules and expands to a more open (nearly extended) structure which further self-assembles to form a supramolecular sheet like structure. However, crystals obtained from chloroform show a supramolecular double helix which is stabilized by intermolecular hydrogen bonding and π–π stacking interactions. The report describes how significantly different self-assembled structures are developed from compounds folded in a subtly different manner by interaction with the solvent.

 Please wait while we load your content...
Please wait while we load your content...