New properties in old systems: cooperative electric order in ferrocene and ammonia-borane†
Abstract
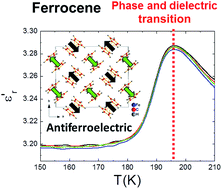
After half a century of intensive and extensive studies on ferrocene [Fe(C5H5)2] and ammonia-borane (H3N·BH3), it is very exciting to observe that these “classical” compounds still hide interesting properties never described before. Despite it being well-known that they both experience phase transitions as a function of temperature, here, for the first time, we give experimental evidence for the dielectric transitions they experience associated to the former, results that we support with DFT calculations. In that context, we report that ferrocene displays a temperature-induced paraelectric to antiferroelectric transition associated with the monoclinic–triclinic transition, which implies order–disorder of the cyclopentadienyl (Cp) ligands and displacement of the Fe atoms within the ferrocene molecules. As for the ammonia-borane, we report a sharp dielectric transition at T ≈ 232 K, associated with a structural transition that combines ordering and atomic displacement of the H3N·BH3 molecules. In this case, we attribute such behaviour to the temperature dependent displacement of the H3N·BH3 molecules out-of the crystal polar c-axis.


 Please wait while we load your content...
Please wait while we load your content...