Catalytic decomposition action of hollow CuFe2O4 nanospheres on RDX and FOX-7
Abstract
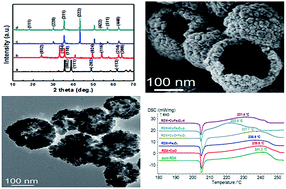
Two hollow CuFe2O4 nanospheres (CuFe2O4-p and CuFe2O4-d) were successfully prepared using a hydrothermal method. CuFe2O4-p has a smaller size and higher BET specific surface area compared to CuFe2O4-d. The catalytic activity of nano-CuO, nano-Fe2O3, physically mixed CuO + Fe2O3, CuFe2O4-p, and CuFe2O4-d on the thermal decomposition of RDX and FOX-7 were comparatively investigated by differential scanning calorimetry (DSC) method. The results showed that CuFe2O4-p and CuFe2O4-d exhibited higher catalytic activity than the others, and the apparent activation energies of decomposition were reduced by 37.7 and 28.4 kJ mol−1 for RDX, 40.1 and 30.5 kJ mol−1 for FOX-7, respectively. The catalytic performance of CuFe2O4-p for the decomposition of RDX and FOX-7 was superior to that of CuFe2O4-d.

 Please wait while we load your content...
Please wait while we load your content...