Study on the efficiency of some amine derivatives as corrosion and scale inhibitors in cooling water systems†
Abstract
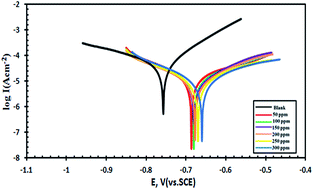
Corrosion and scaling are the main problems in cooling water systems. To address these problems, this paper aimed to study the inhibition efficiency of some amine derivatives for carbon steel in sea water using electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The selected compounds, with different structures and sizes, were found to be adsorbed onto the carbon steel surface and had good performance as corrosion and scale inhibitors. Potentiodynamic polarization studies indicate that the amine derivatives act as mixed type corrosion inhibitors, while the data obtained from EIS revealed that the value of the charge transfer resistance (Rt) increased when increasing the inhibitor concentration leading to an increase in the inhibition efficiency. Also, the obtained results from this study show that the selected amines can decrease scale build-up growth under the experimental conditions. The surface morphology of the carbon steel was investigated in the absence and presence of the selected inhibitors through scanning electron microscopy (SEM) and energy dispersive analysis of X-rays.

 Please wait while we load your content...
Please wait while we load your content...