l-Proline nitrate: a recyclable and green catalyst for the synthesis of highly functionalized piperidines†
Abstract
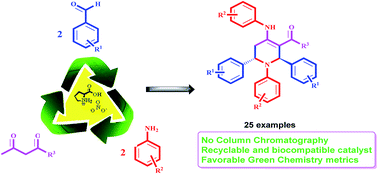
The synthesis of highly functionalized piperidines has been strategically accessed via organo-catalytic three components (in situ five components) reaction of an amine, aldehyde and 1,3-dicarbonyl compound. This imine based multi-component reaction was realized using fully green L-proline nitrate as recyclable room temperature ionic liquid. Recycling of the catalyst was possible up to five runs without loss of catalyst activity. Smaller E-factor (0.255) and process mass intensity (PMI = 3.35), high atom-economy (AE = 89.5%) and reaction mass efficiency (RME = 79.66%) demonstrates the higher environmental compatibility and sustainability of this protocol. DFT calculations showed that the L-proline catalyzed reaction proceeds by three pathways; (i) via proline enamine pathway, (ii) proline mediated aniline enamine pathway or (iii) the pathway involving iminium activation of the aldehyde to provide the Knoevenagel product.

 Please wait while we load your content...
Please wait while we load your content...