Crystalline arrays of molecular rotors with TIPS-trityl and phenolic-trityl stators using phenylene, 1,2-difluorophenylene and pyridine rotators†
Abstract
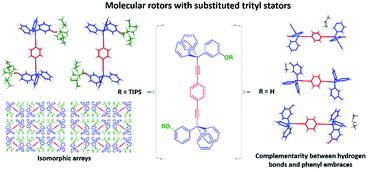
In this work, we describe the synthesis and solid-state characterization of a series of molecular rotors with tri-isopropylsilyloxy-substituted (TIPS) trityl stators axially linked to 1,4-diethynylphenylene, 3,6-diethynyl-1,2-difluorophenylene and 2,5-diethynylpyridine rotators to produce 1,4-bis[(3,3-diphenyl-3-(3′-(tri-isopropylsilyloxy)-phenyl)-prop-1-yn-1-yl)]benzene (1), 1,4-bis[(3,3-diphenyl-3-(3′-(tri-isopropylsilyloxy)-phenyl)-prop-1-yn-1-yl)]-2,3-difluorobenzene (2) and 2,5-bis[(3,3-diphenyl-3-(3′-tri-isopropylsilyloxy)-phenyl)-prop-1-yn-1-yl)]pyridine (3). The subsequent removal of the TIPS protecting group led to their corresponding hydroxyl-substituted trityl derivatives (4) and (5). TIPS- and HO-substituted stators are involved in different inter- and intramolecular interactions (hydrogen bonding, phenyl embraces, C–H–π interactions) that give rise to isomorphic packing motifs that constrained the rotational dynamics in the solid-state to the slow exchange regime.

 Please wait while we load your content...
Please wait while we load your content...