Double functionalization of 2-amino-2′-hydroxy-1,1′-biaryls: synthesis of 4-nitro-dibenzofurans and benzofuro-indoles†
Abstract
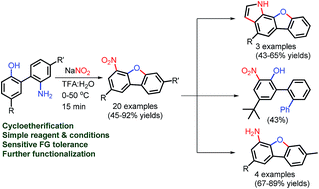
Several substituted 2′-amino-biphenyl-2-ols were synthesized. The mild and TM-free double functionalization: nitration and cycloetherification of 2′-amino-biphenyl-2-ols have been achieved for the synthesis of functionalized 4-nitro-dibenzofurans utilizing NaNO2 in TFA and water. Interestingly, nitration of phenol ring was observed along with the dibenzofuran ring formation in the one-pot synthesis. The position of the nitro group in several dibenzofurans is also established by X-ray crystal structure studies. Further functionalization of the obtained 4-nitro-dibenzofurans has been carried into 3-nitro-terphenyl-2-ol, novel benzofuro-indoles, and amino-dibenzofurans. The mechanistic understanding on double functionalization of 2′-amino-biphenyl-2-ols suggests that reaction proceeds by a combination of nitration of the phenol ring followed by Sandmeyer reaction for dibenzofuran ring formation.


 Please wait while we load your content...
Please wait while we load your content...