Ion exchange synthesis of an all tungsten based Z-scheme photocatalytic system with highly enhanced photocatalytic activity†
Abstract
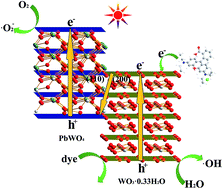
The first example of an all tungsten based Z-scheme photocatalyst (composed of WO3·0.33H2O and PbWO4) has been synthesized via an ion exchange method. The composites exhibit much higher photocatalytic activities than those of individual hydrated tungsten trioxide and lead tungstate. The apparent photodegradation rate constants of RhB over the composites are more than 6 to 8 times higher than those of the two monomer components. After 30 min, the RhB can be removed almost completely by the composite, while only 30% RhB and 35% RhB can be, respectively, eliminated for the two single components. In addition, the composite photocatalyst displays excellent activity in the photodegradation of methyl orange (MO). The relationship between the two monomer components has been investigated using XRD, Raman, XPS, SEM, and TEM measurements. The hydrated tungsten trioxide is formed on the surface of lead tungstate via a complex process that includes ion exchange and crystal structure rearrangement. Ion exchange occurs at the end of the (110) facet of lead tungstate. The results of photocatalytic mechanism and photodeposition Pt experiments indicate that the excellent photocatalytic activity of the composite photocatalyst is due to the advantage of its Z-scheme configuration. This study suggests that highly effective Z-scheme photocatalysts can be built by ion exchange.

 Please wait while we load your content...
Please wait while we load your content...