Chloroquinoline–acetamide hybrids: a promising series of potential antiprotozoal agents†
Abstract
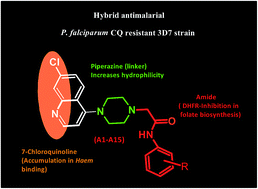
In an endeavour to develop efficacious antiprotozoal agents 2-[4-(7-chloroquinolin-4-yl)piperazin-1-yl]acetamide derivatives were synthesized and screened in vitro against the HM1 : IMSS strain of E. histolytica and 3D7 strain of P. falciparum. Among the twenty-seven synthesized compounds, eleven evinced propitious anti-amoebic activity with IC50 values ranging from 0.41 to 1.80 μM) lower than the standard drug metronidazole (IC50 1.80 μM). All the compounds inhibited the in vitro growth of P. falciparum (IC50 range: 0.30–33.52 μM). Compounds A22 and A25 were found to be the most active antimalarial derivatives, and compound A16 the most active in inhibiting β-haematin formation; however compound A25 displayed the more favourable safety profile. The crystal structure for the compounds A7, A8, A12 and A21 was also determined. The molecular docking of crystal resolved inhibitors with PfDHFR allowed identification of stabilizing interactions within enzyme active sites. These compounds affirm the potential for further derivatives to enhance antiprotozoal activity whilst retaining their safety profile.

 Please wait while we load your content...
Please wait while we load your content...