Kinetic exploration supplemented by spectroscopic and molecular docking analysis in search of the optimal conditions for effective degradation of malachite green†
Abstract
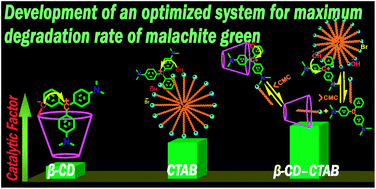
The degradation of malachite green (MG) by an alkaline hydrolytic process has been explored spectrophotometrically. The kinetics of the reaction have been meticulously studied under the influence of cationic alkyltrimethylammonium bromide (DTAB, TTAB and CTAB) surfactants, α-, β- and γ-cyclodextrins (CDs) and surfactant–β-CD mixed systems applying pseudo-first order conditions at 298 K. The surfactants and cyclodextrins individually catalyze the hydrolytic rate, whereas surfactant–β-CD mixed systems exhibit both an inhibiting and catalytic influence depending on the surfactant concentrations. The kinetic results have been explained precisely based on the pseudo-phase ion exchange (PIE) model of micelles and CD-catalyzed model of CD systems. The surfactants exhibit micellar surface catalysis, while CDs accelerate the rate by forming MG–CD inclusion complexes, thereby facilitating nucleophilic attack of its ionized secondary hydroxyl group on the carbocation center of MG. The encapsulation of MG within the supramolecular host cavity of the CDs has been investigated diligently using a steady-state absorption spectroscopic technique. The result shows 1 : 1 host–guest complexation with different relative orientations of the guest (MG) inside the hosts. Studies employing density functional theory (DFT) as well as molecular docking analysis provide valuable insight on the insertion mechanism. The results reveal that quantitative analysis can be utilized to predict the optimum conditions for the fastest degradation of MG in ambient environments.

 Please wait while we load your content...
Please wait while we load your content...