Nature of reactant and influence of water on the supramolecular patterns and luminescent properties of organic salts comprising (1,1′-biphenyl)-4,4′-disulfonate and triphenylmethanaminium†
Abstract
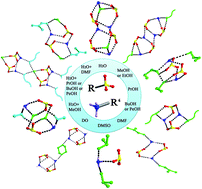
The solvent reaction of 1,1′-biphenyl-4,4′-disulfonic acid (H2BPDSA) and triphenylmethylamine (TPMA) gives rise to fourteen supramolecular compounds, namely, 2(HTPMA)+·(BPDSA)2−·(H2O) (1), 2(HTPMA)+·(BPDSA)2−·(MeOH) (2), 2(HTPMA)+·(BPDSA)2−·(EtOH) (3), 2(HTPMA)+·(BPDSA)2−·3(n-PrOH) (4), 2(HTPMA)+·(BPDSA)2−·2(n-BuOH) (5), 2(HTPMA)+·(BPDSA)2−·2(n-PeOH) (6), 2(HTPMA)+·(BPDSA)2−·4(DMF) (7), 2(HTPMA)+·(BPDSA)2−·4(DMSO) (8), 2(HTPMA)+·(BPDSA)2−·(DO) (9), 2(HTPMA)+·(BPDSA)2−·2(H2O)·2(MeOH) (10), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-PrOH) (11), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-BuOH) (12), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-PeOH) (13) and 2(HTPMA)+·(BPDSA)2−·4(H2O)·2(DMF) (14) (DO = 1,4-dioxane), which have been characterized by elemental analysis, IR, TG, PL, and powder and single-crystal X-ray diffraction. Structural analyses indicate that salts 1–14 show seven types of packing diagram. Moreover, salts 1–9 present regular supramolecular structural variations with the change of the nature of the solvent molecules. Salts 1–3 exhibit double chain motifs with small molecules of H2O, MeOH and EtOH. Salts 4–7 present single chain structures with larger n-PrOH, n-BuOH, n-PeOH and DMF molecules. Salt 8 only exhibits a simple discrete motif owing to the triangular pyramid configuration of the DMSO molecule. In contrast, the DO molecules in salt 9 have two opposite acceptor O atoms, which extend adjacent HTPMA+ cations and BPDSA2− dianions into a (4,4) layer motif. Furthermore, the involvement of water molecules could modulate the dimension level of supramolecular patterns according to the different hydrogen bonding modes of H2O molecules. Salts 10 and 14 present different double and single chain structures from the single chains in salts 2 and 7 owing to the HB′3 and HB′2 modes of H2O molecules. Similarly, salts 11–13 show different (4,4) layer motifs from the chain structures in salts 4–6 with the assistance of the H2O molecules in HB4 mode. Luminescence investigations reveal that the emission maximum of salts 1–9 varies from 343 to 358 nm. In comparison, salts 10 and 14 show stronger emission intensity, whereas salts 12 and 13 show weaker emission intensity than their corresponding anhydrous salts owing to the structure transformations induced by the involvements of water molecules.

 Please wait while we load your content...
Please wait while we load your content...