Room temperature deep eutectic solvents of (1S)-(+)-10-camphorsulfonic acid and sulfobetaines: hydrogen bond-based mixtures with low ionicity and structure-dependent toxicity†
Abstract
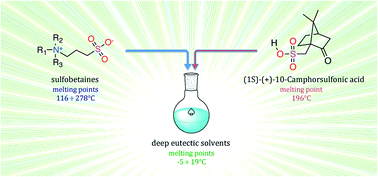
Twelve novel deep eutectic solvents (DESs) were prepared and characterized in this work. They are mixtures of (1S)-(+)-10-camphorsulfonic acid (CSA) and differently structured sulfobetaines (SBs) with aliphatic, aromatic and amphiphilic moieties. They are liquids at room temperature, their melting points span, in fact, from −5° to 19 °C, so we can name these mixtures RTDESs (room temperature deep eutectic solvents). These zwitterionic DESs were characterized in terms of their viscosity, conductivity (and therefore ionicity via Walden plots), density, surface tension and toxicity on eukaryotic model cells. The collected data suggest that the interaction between CSA and the SBs can be ascribed as a hydrogen bond instead of a proton transfer, therefore they are not ionic liquids. To our knowledge, their position on the Walden plot, in the left portion close to the diagonal, has not yet been observed for other DESs or ionic liquid systems and indicates the low ionicity of these mixtures. A FTIR-based bioassay was performed to determine the toxicity of these mixtures on eukaryotic model cells (Saccharomyces cerevisiae). The DESs showed merely a dehydrating effect on the cells, similar to that produced by CaCl2, a low cell toxicity salt. This supports these DESs as promising green media. Amphiphilic SBs DESs showed a stronger effect on the cells and a structure-activity trend can be described for this class. A preliminary study on the use of these novel DESs as Brønsted catalyst media was accomplished by the use of one of them in chalcone synthesis, which showed promising catalytic and recycling capabilities.

 Please wait while we load your content...
Please wait while we load your content...