Extractive desulfurization of fuel using N-butylpyridinium-based ionic liquids†
Abstract
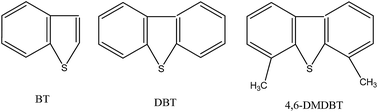
Sulfur compounds in fuels have become one of the sources of serious environmental problems. The extractive desulfurization using ionic liquids (ILs) has attracted great attention in recent years. In this work, the pyridinium-based ionic liquids (ILs) N-butylpyridinium thiocyanate ([C4Py][SCN]), N-butylpyridinium bis(trifluoromethylsulfonyl)imide ([C4Py][NTf2]), and N-butylpyridinium dicyanamide ([C4Py][N(CN)2]) were used as extractants for desulfurization of model fuels. The results demonstrate that the structure of the anion influences the extractive performance of ILs, following the order of [NTf2] < [SCN] < [N(CN)2], which was proved by the electrostatic interaction between ions and DBT through molecular dynamics simulations (MD). In addition, the selectivity of sulfur compounds by the extraction process followed the order of dibenzothiophene (DBT) > benzothiophene (BT) > 4,6-dimethyldibenzothiophene (4,6-DMDBT). Moreover, the [C4Py][N(CN)2] can be recycled at least 4 times with little decrease in the desulfurization activity.
- This article is part of the themed collection: Ionic Liquids: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...