Synthesis and steroid sulfatase inhibitory activities of N-alkanoyl tyramine phosphates and thiophosphates†
Abstract
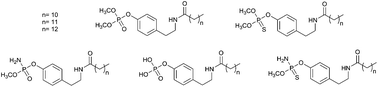
A series of phosphate and thiophosphate analogs based on the frameworks of N-alkanoyl tyramines have been synthesized and biologically evaluated. Their binding modes have been modeled using docking techniques. The inhibitory effects of the synthesized compounds were tested on STS isolated from the human placenta as well as the MCF-7, MDA-MB-231 and SkBr3 cancer cell lines. Most of the new STS inhibitors possessed potent activity against STS. In the course of our investigation, 4-(2-dodecanoylamino-ethyl)-phenyl dimethyl phosphate 4a demonstrated the greatest inhibitory effect, with IC50 values of 0.39 μM (IC50 value of 15.44 μM for the 4-(2-dodecanoylamino-ethyl)-phenyl sulfamate used as a reference). The compound 4a exhibited the highest potency against the MCF-7, MDA-MB-231 and SkBr3 cancer cell lines, with a GI50 values of 8.80, 6.48 and 5.76 μM, respectively. The structure–activity relationships of the synthesized phosphate- and thiophosphate-based tyramine derivatives with the STS enzyme are discussed.

 Please wait while we load your content...
Please wait while we load your content...