Structure, stability and intramolecular interaction of M(N5)2 (M = Mg, Ca, Sr and Ba) : a theoretical study†
Abstract
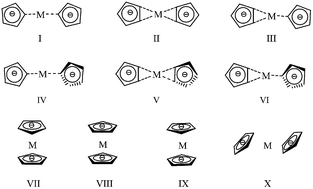
The potential energetic materials, alkaline earth metal complexes of the pentazole anion (M(N5)2, M = Mg2+, Ca2+, Sr2+ and Ba2+), were studied using the density functional theory. The stable conformation, stability, and pyrolysis mechanism of these complexes were predicted. Dissociation of the title complexes consists of sequential breaking of two N5 rings and the required activation energies (Ea,1, Ea,2 in kJ mol−1) increase in the order of Mg(N5)2 (97.7, 99.9) < Ca(N5)2 (102.3, 104.0) < Sr(N5)2 (105.2, 106.1) < Ba(N5)2 (106.9, 108.8). The small magnitudes (10−7 to 10−4) of the reaction rates show that the dissociation reactions of two N5 rings are very slow. Less electron transfer between the N5 ring and the metal, stronger covalent bonding interactions, and stronger dispersion interactions are responsible for the more stable complexes. The negative change in the enthalpy of dissociation reaction and the acceptable stability illustrate that these complexes may be used as high energy materials.

 Please wait while we load your content...
Please wait while we load your content...