Diastereoselective synthesis of functionalised carbazoles via a sequential Diels–Alder/ene reaction strategy†
Abstract
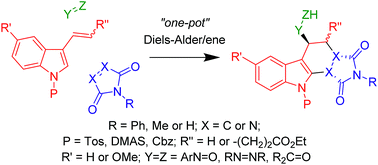
An operationally simple one-pot, three-component, diastereoselective synthesis of saturated carbazoles and related pyridazino[3,4-b]indoles, based on two sequential intermolecular pericyclic reactions, is described. The reaction sequence involves an intermolecular Diels–Alder (D–A) reaction of a 3-vinyl-1H-indole, containing an electron withdrawing N-protecting group, with a suitable dienophile. Due to the electron withdrawing nature of the N-protecting group the resultant D–A cycloadducts are sufficiently stabilised to allow for a subsequent in situ diastereospecific intermolecular ene reaction to take place with an added enophile, generating functionalised carbazoles with relative stereocontrol of up to four stereocentres.

 Please wait while we load your content...
Please wait while we load your content...