Investigation of the influence of alkyl side chain length on the fluorescence response of C153 in a series of room temperature ionic liquids†
Abstract
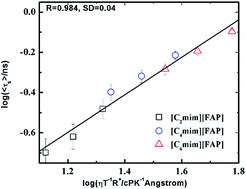
The fluorescence response of coumarin 153 (C153) has been investigated in a series of 1-alkyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate room temperature ionic liquids (RTILs) with systematic variation of alkyl chain length (ethyl, butyl and hexyl) to examine the effect of the alkyl side chain length of the cationic moiety on solute and solvent relaxation dynamics. Physicochemical properties associated with the present RTILs are estimated at different temperatures. While the viscosity values increase with increasing alkyl chain length, density values decrease with increasing length of alkyl side chain. Steady state fluorescence measurements reveal that C153 experiences more nonpolar microenvironments with an increase in the alkyl chain length. Time resolved studies have demonstrated that the lengths of alkyl side chain have a noticeable role in governing the solvation dynamics in these media. It has been observed that the average solvent relaxation time estimated for these RTILs can be better correlated when both the size of the alkyl chains and the bulk viscosity of the respective RTILs are considered. Interestingly, apart from the viscosity effect, a negligible influence of alkyl chain length has been observed for rotational diffusion of C153.

 Please wait while we load your content...
Please wait while we load your content...