Chiral, fluorescent microparticles constructed by optically active helical substituted polyacetylene: preparation and enantioselective recognition ability†
Abstract
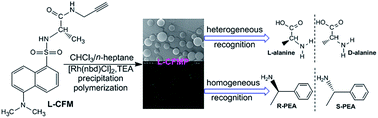
Microparticles simultaneously showing optical activity and fluorescence were prepared based on fluorescent, optically active helical polymers. Chiral and fluorescent substituted acetylene monomers (L- and D-CFM) were synthesized and then underwent precipitation polymerization in a solvent mixture of CHCl3/n-heptane in the presence of a Rh catalyst at room temperature. The CHCl3/n-heptane mixture at a suitable ratio provided spherical microparticles (L- and D-CFMPs) with uniform diameters of 910 nm in a high yield (ca. 90 wt%). The microparticles were comprised of polymer chains (number-average molecular weight, 8700 g mol−1) that were found to adopt chiral helical structures, according to circular dichroism and UV-vis absorption spectroscopy. The fluorescence property was measured using fluorescent microscopy and spectroscopy. Remarkably, the novel microparticles exhibited enantioselective recognition ability towards alanine and phenylethylamine enantiomers. However, L- and D-CFMPs behaved differently in the enantioselective recognition processes. Possible mechanisms were proposed for the observed enantioselective recognition.

 Please wait while we load your content...
Please wait while we load your content...