Application of chiral ligands: carbohydrates, nucleoside-lanthanides and other Lewis acid complexes to control regio- and stereoselectivity of the dipolar cycloaddition reactions of nitrile oxides and esters†
Abstract
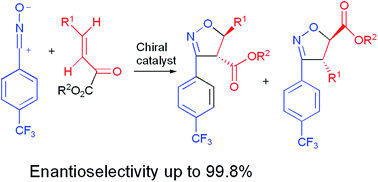
Chiral Lewis acid mediated 1,3-dipolar cycloaddition reactions of 4-trifluoromethylbenzonitrile oxide to methyl crotonate as well to β-substituted acrylates and (Z)-pent-2-en-1-yl esters were examined. Excellent enantioselectivities with moderate to good regioselectivities were achieved for crotonates with complexes of BiBr3 with (+)-(4,6-benzylidene)methyl-α-D-glucopyranoside C, with the L-ascorbic acid I–FeCl3 system, and with lipase Candida antarctica. High enantiomeric excess was observed for isopropyl ester and benzyl ester. The outstanding ee values were achieved for acrylates with β-t-butyl, cyclohexyl, and 1,3-benzodioxol-5-yl groups in cycloadditions catalyzed by C–Yb(OTf)3 and the (+)-2-hydroxy-3-pinanone N–TiCl4 system. High enantioselectivities were found in reactions of (Z)-pent-2-en-1-yl esters mediated by complexes N–Mg(OTf)2 and N–TiCl4.
- This article is part of the themed collection: RSC Advances Editors' collection: f Block Chemistry

 Please wait while we load your content...
Please wait while we load your content...