Impact of linker in polypyrrole/quinone conducting redox polymers†
Abstract
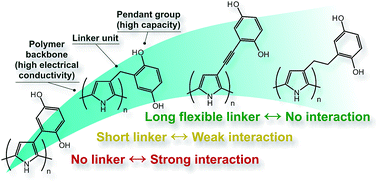
Organic conducting redox polymers are being investigated as the active component for secondary battery applications, as they have the potential to solve two of the main problems with small molecule-based organic electrodes for electrical energy storage, viz dissolution of the active compound in the electrolyte, and slow charge transport through the material. Herein we report the synthesis of a series of conducting redox polymers based on polypyrrole with hydroquinone pendant groups that are attached to the backbone via different linkers, and we investigate the impact of the linker on the interaction between the backbone and the pendant groups. For the directly linked polymer, oxidation of the pendant groups leads to a decrease of bipolaron absorbance, as well as a decrease in mass of the polymer film, both of which are reversible. The origin of these effects is discussed in light of the influence of the linker unit, electrolyte polarity, and electrolyte salt. For the longest linkers in the series, no interaction was observed, which was deemed the most beneficial situation for energy storage applications, as the energy storage capacity of the pendant groups can be utilized without disturbing the conductivity of the polymer backbone.
- This article is part of the themed collection: Polymers for Electrochemical Energy Storage

 Please wait while we load your content...
Please wait while we load your content...