One-pot hydrothermal synthesis of hematite-reduced graphene oxide composites for efficient removal of malachite green from aqueous solution†
Abstract
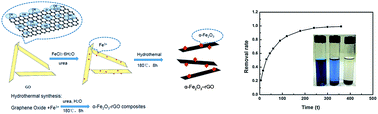
The facile one-pot synthetic route to prepare a 3D graphene composition of hematite (α-Fe2O3)–reduced graphene oxide (rGO) hybrid materials has been reported. The α-Fe2O3–rGO materials exhibit excellent capacity to remove malachite green (MG) from water. The pristine suspension of graphene oxide (GO) from the Hummers method mixed with FeCl3 and urea in the solution, is in situ transformed into α-Fe2O3–rGO composites under hydrothermal conditions. The morphology and structure of the α-Fe2O3–rGO composites are characterized using transmission electron microscopy, X-ray diffraction, Raman spectroscopy, X-ray photoemission spectroscopy, Fourier transform-infrared spectroscopy, etc. It is found that α-Fe2O3 nanoparticles with cubic shapes and particles with the cubic side of 10–30 nm are uniformly distributed on the graphene layer. The application of α-Fe2O3–rGO materials for the removal of MG from the aqueous solutions is investigated. The Langmuir model is found to fit well with the experimental isotherm data, with a maximum adsorption capacity of 438.8 mg g−1 for MG dye. The MG adsorption process is controlled by the pseudo-second-order rate model. The excellent capacity of α-Fe2O3–rGO to remove MG from water is ascribed to the synergetic adsorptive effect between α-Fe2O3 and rGO. The research provides an attractive adsorbent for removing the hazardous materials from wastewater.

 Please wait while we load your content...
Please wait while we load your content...