The effect of surfactant type and concentration on the size and stability of microbubbles produced in a capillary embedded T-junction device
Abstract
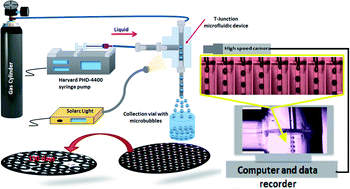
This work presents an investigation of the effect of various surfactants on microbubble formation, size and stability in a capillary embedded T-Junction microfluidic device. Four different surfactants were chosen. An anionic surfactant, sodium dodecyl sulfate (SDS), two non-ionic surfactants, polyoxyethylene sorbitan monopalmitate (Tween 40) and polyoxyethylene glycol 40 stearate (PEG 40), and a cationic surfactant, cetyltrimethyl ammonium bromide (CTAB). Each surfactant was added to 50 wt% aqueous glycerol solution at high concentration (above the critical micelle concentration) varying from 2 to 5 and 10 wt%. Static surface tension and contact angle were measured, as well as the viscosity of the solutions. While the value of surface tension did not significantly change with increasing surfactant concentration, other properties of the solutions (i.e. viscosity and contact angle) were affected. Microbubbles with sizes varying from 50 to 360 μm all with polydispersity index values of <2% were produced with this technique. The nonionic surfactants were found to produce smaller bubbles. This is likely to have been due to their higher adsorption on to the hydrophobic channel surface and hence increase in the thickness of the liquid film at the contact line between the three phases for approximately similar capillary numbers and viscosities. Bubble stability for all cases was evaluated by monitoring the change in average diameter with time. Microbubbles coated with PEG 40 were found to be the most stable, lasting for 150 days with a uniform size reduction of ∼1.5% as compared with SDS microbubbles lasting only for 30 min after collection.

 Please wait while we load your content...
Please wait while we load your content...