Triazolium based ionic liquid crystals: effect of asymmetric substitution†
Abstract
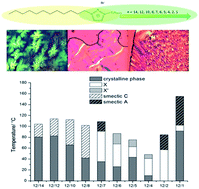
A new series of ten different asymmetrical 1-dodecyl-3-alkyl-triazolium bromides, [C12CnTr][Br], has been synthesized and their mesomorphic behavior studied by DSC (differential scanning calorimetry), POM (polarizing optical microscopy) and SAXS (small angle X-ray scattering). The influence of the chain length of the triazolium salts is investigated to explore the effect of asymmetric substitution on the phase behaviour of these compounds. For that reason, the length of one alkyl chain was varied from 14 to 1 carbon atoms (n = 14, 12, 10, 8–4, 2, 1) while the other alkyl chain was kept at 12 carbon. Single crystal X-ray structure analysis of compounds [C12C12Tr][Br] and [C12C5Tr][Br] reveal that the cations adopt a U-shaped conformation with head-to-head arranged triazolium cores. In contrast, for [C12C1Tr][Br], a rod like shape of the cation with interdigitated alkyl chains is found. All investigated compounds are thermotropic liquid crystals. Higher ordered smectic phases, smectic C as well as smectic A phases were found depending on the chain length of the cation. Overall the clearing point temperature decreases with decreasing chain length with exception for the n-dodecyl-3-alkyltrizoliumbromides with the two shortest alkyl chains, [C12C2Tr][Br] and [C12C1Tr][Br], which present higher clearing temperatures (86 and 156 °C) and are structurally distinctly different.
- This article is part of the themed collection: Ionic Liquids: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...