A novel approach to alginate aerogels: carbon dioxide induced gelation
Abstract
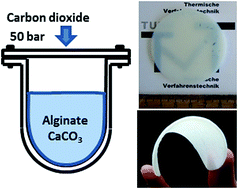
A novel technique for the preparation of alginate aerogels, that utilizes high pressure CO2, is presented. In pressurized carbon dioxide at 5 MPa and 298 K, a suspension of calcium carbonate dispersed in a sodium alginate solution undergoes irreversible gelation without additional pH modifiers or crosslinkers. Solvent exchange to ethanol at ambient conditions followed by supercritical drying with carbon dioxide resulted in alginate aerogels with remarkable properties compared to the state of the art. They are very light with densities down to 0.06 ± 0.02 g cm−3, translucent and possess a fibrillar structure with both meso- and macroporosity. For selected samples, the surface area and mesopore volume are found to be 545 ± 77 m2 g−1 and 6.98 cm3 g−1, respectively. Thermal conductivity measurements using the hot-wire method revealed excellent thermal insulation properties: thermal conductivity was determined to be in the range of (18–22) ± 2 mW m−1 K−1. Although the initial aerogels are flexible to a certain extent, they can be classified as viscoplastic materials and their compression indicates improved flexibility and retained mesoporosity. A possible approach for the production of alginate aerogels in an integrated one-pot process – that combines gelation, solvent exchange and supercritical drying – is outlined.

 Please wait while we load your content...
Please wait while we load your content...