Enhanced shape memory performance of polyurethanes via the incorporation of organic or inorganic networks†
Abstract
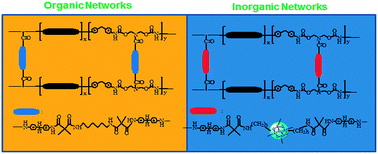
A diol compound with a reactive azetidine-2,4-dione group was prepared and introduced as a side chain moiety of poly(ε-caprolactone) (PCL) based polyurethane (PU). The PUs with reactive pendants were crosslinked by 1,6-diaminohexane, or modified by an alkoxysilane (3-aminopropyltriethoxysilane, APTS) followed by a sol–gel reaction to bring about crosslinked PUs for shape memory applications. Thermal and mechanical properties of the crosslinked PUs are strongly dependent on the chemical structure of the interchain-linker between the polymer chains. Higher tensile strengths would be achieved for the PU samples crosslinked with alkoxysilanes in comparison with the ones crosslinked with an aliphatic diamine, while higher values of elongation at break were observed for the latter. As compared to the PCL based linear and side-chain PUs, much better shape memory performance was observed as the PU sample was crosslinked with the aliphatic diamine, or by the sol–gel reaction of alkoxysilanes, particularly the latter. Higher shape retention (91%) and recovery (99%) ratios were observed for the CPU samples with inorganic networks. By introducing the chemically crosslinked structures, the deformed PU samples completely recovered their original shape in less than 10 seconds without any deficiency during shape recovery measurements.

 Please wait while we load your content...
Please wait while we load your content...