Brønsted acid-catalyzed hydroarylation of activated olefins†
Abstract
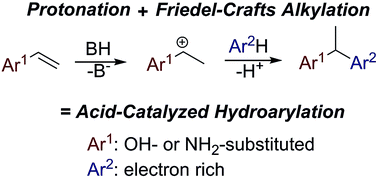
A mild, regiospecific Brønsted acid-catalyzed hydroarylation of activated olefins, capable of the formation of quinone methide-like intermediates, has been investigated. Variously substituted 2- and 4-vinylphenols, 4-vinylaniline or 6-vinyl-naphthalen-2-ol were successfully implemented in a sequential protonation and Friedel–Crafts-type alkylation reaction of electron-rich arenes.

 Please wait while we load your content...
Please wait while we load your content...