Malachite green adsorption onto Fe3O4@SiO2-NH2: isotherms, kinetic and process optimization†
Abstract
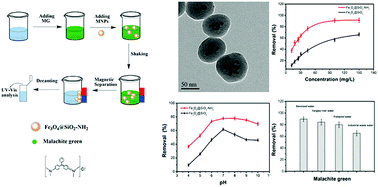
Higher environmental standards have made the removal of dye from water an important problem for many industrialized countries. Among the removal methods, magnetic adsorbents have received much attention for adsorption of dyes. In this paper, a novel magnetic adsorbent with an amino-modified SiO2-coated Fe3O4 magnetite was prepared. The analysis of Fe3O4@SiO2-NH2 proved that the nanoparticles were uniform with a diameter of 90 nm. The difference between Fe3O4@SiO2-NH2 and Fe3O4@SiO2 on the adsorption behaviour of malachite green was studied. Fe3O4@SiO2-NH2 showed a good capacity for the adsorption of malachite green compared with Fe3O4@SiO2, which was shown to be influenced by the initial concentration and pH. Under the optimal conditions, the removal efficiency of malachite green was over 90% by Fe3O4@SiO2-NH2, while using Fe3O4@SiO2 efficiency was below 60%. The experimental data fit well with the Freundlich isotherm and the pseudo-second-order kinetic model. In further applications, real samples were treated with Fe3O4@SiO2-NH2 nanoparticles. All the results demonstrated that the Fe3O4@SiO2-NH2 nanoparticles could be promising and effective adsorbents.

 Please wait while we load your content...
Please wait while we load your content...